Publications
- Läubli, N. F., Burri, J. T., Marquard, J., Vogler, H., Mosca, G., Vertti-Quintero, N., Shamsudhin, N., deMello, A., Grossniklaus, U., Ahmed, D., Nelson, B. J. 3D mechanical characterization of single cells and small organisms using acoustic manipulation and force microscopy. Nature Communications, 2021. doi: 10.1038/s41467-021-22718-8
Quantitative micromechanical characterization of single cells and multicellular tissues or organisms is of fundamental importance to the study of cellular growth, morphogenesis, and cell-cell interactions. However, due to limited manipulation capabilities at the microscale, systems used for mechanical characterizations struggle to provide complete three-dimensional coverage of individual specimens. Here, we combine an acoustically driven manipulation device with a micro-force sensor to freely rotate biological samples and quantify mechanical properties at multiple regions of interest within a specimen. The versatility of this tool is demonstrated through the analysis of single Lilium longiflorum pollen grains, in combination with numerical simulations, and individual Caenorhabditis elegans nematodes. It reveals local variations in apparent stiffness for single specimens, providing previously inaccessible information and datasets on mechanical properties that serve as the basis for biophysical modelling and allow deeper insights into the biomechanics of these living systems.
- Hernandez-Lagana, E.*, Mosca, G.*, Mendocilla-Sato, E.*, Pires, N., Frey, A., Giraldo-Fonseca, A., Michaud, C., Grossniklaus, U., Hamant, O., Godin, C., Boudaoud, A., Grimanelli, D., Autran, D., Baroux, C. Organ geometry channels reproductive cell fate in the Arabidopsis ovule primordium. eLife, 2021. doi: 10.7554/eLife.66031
In multicellular organisms, sexual reproduction requires the separation of the germline from the soma. In flowering plants, the female germline precursor differentiates as a single spore mother cell (SMC) as the ovule primordium forms. Here, we explored how organ growth contributes to SMC differentiation. We generated 92 annotated 3D images at cellular resolution in Arabidopsis. We identified the spatio-temporal pattern of cell division that acts in a domain-specific manner as the primordium forms. Tissue growth models uncovered plausible morphogenetic principles involving a spatially confined growth signal, differential mechanical properties, and cell growth anisotropy. Our analysis revealed that SMC characteristics first arise in more than one cell but SMC fate becomes progressively restricted to a single cell during organ growth. Altered primordium geometry coincided with a delay in the fate restriction process in katanin mutants. Altogether, our study suggests that tissue geometry channels reproductive cell fate in the Arabidopsis ovule primordium.
- Long, Y., Cheddadi, I., Mosca, G., Mirabet, V., Dumond, M., Kiss, A., Traas, J., Godin, C., Boudaoud, A. Cellular Heterogeneity in Pressure and Growth Emerges from Tissue Topology and Geometry. Current Biology, 2020. doi: 10.1016/j.cub.2020.02.027
Cell-to-cell heterogeneity prevails inmany systems, as exemplified by cell growth, although the origin and function of such heterogeneity are often unclear. In plants, growth is physically controlled by cell wall mechanics and cell hydrostatic pressure, alias turgor pressure. Whereas cell wall heterogeneity has received extensive attention, the spatial variation of turgor pressure is often overlooked. Here, combining atomic force microscopy and a physical model of pressurized cells, we show that turgor pressure is heterogeneous in the Arabidopsis shoot apical meristem, a population of stem cells that generates all plant aerial organs. In contrast with cell wall mechanical properties that appear to vary stochastically between neighboring cells, turgor pressure anticorrelates with cell size and cell neighbor number (local topology), in agreement with the prediction by our model of tissue expansion, which couples cell wall mechanics and tissue hydraulics. Additionally, our model predicts two types of correlations between pressure and cellular growth rate, where high pressure may lead to fasteror slower-than-average growth, depending on cell wall extensibility, yield threshold, osmotic pressure, and hydraulic conductivity. The meristem exhibits one of these two regimes, depending on conditions, suggesting that, in this tissue, water conductivity may contribute to growth control. Our results unravel cell pressure as a source of patterned heterogeneity and illustrate links between local topology, cell mechanical state, and cell growth, with potential roles in tissue homeostasis.
- Natonik-Białoń, S.*, Borowska-Wykręt, D.*, Mosca, G., Grelowski, M., Wrzalik, R., Smith, R. S., Kwiatkowska. D. Deformation of a cell monolayer due to osmotic treatment: a case study of onion scale epidermis. Botany, 2020 doi: 10.1139/cjb-2019-0027
We performed a combination of experiments and mechanical simulations to assess the importance of cell geometry and wall structure in tissue and cell mechanics. Osmotic treatments combined with live imaging were used to quantify deformations at the tissue, cellular, and subcellular levels. We used the adaxial epidermis of onion scale as a model system. We found that the osmotically induced surface strain in onion is small because outer periclinal walls are thick and stiff, requiring bending stiffness to be considered in our mechanical models. As expected, the mechanical behaviors of the tissue and its component cells are related. Upon changes in internal pressure, cells embedded in the tissue undergo deformation that is different from isolated cells, while the tissue undergoes a somewhat counterintuitive deformation, e.g., shrinking upon pressurization, that depends on cell geometry. At the subcellular level, the amount of deformation and its anisotropy vary within the walls of individual cells, and are affected by the cell shape and vicinity of three-way wall junctions. When the turgor pressure is lost, the protoplast-facing wall surface wrinkles due to buckling, with the pattern of wrinkles depending on the strain anisotropy and the local wall geometry.
- Kierzkowski, D.*, Runions, A.*, Vuolo, F., Strauss, S., Lymbouridou, R., Routier-Kierzkowska, AL., Wilson-Sánchez, D., Jenke, A., Galinha, C., Mosca, G., Zhang, Z., Canales, C., Dello Ioio, R., Huijser, P., Smith, R. S., Tsiantis. M. A Growth-Based Framework for Leaf Shape Development and Diversity. Cell, 2019 doi: 10.1016/j.cell.2019.05.011
How do genes modify cellular growth to create morphological diversity? We study this problem in two related plants with differently shaped leaves: Arabidopsis thaliana (simple leaf shape) and Cardamine hirsuta (complex shape with leaflets). We use live imaging, modeling, and genetics to deconstruct these organ-level differences into their cell-level constituents: growth amount, direction, and differentiation. We show that leaf shape depends on the interplay of two growth modes: a conserved organ-wide growth mode that reflects differentiation; and a local, directional mode that involves the patterning of growth foci along the leaf edge. Shape diversity results from the distinct effects of two homeobox genes on these growth modes: SHOOTMERISTEMLESS broadens organ-wide growth relative to edge-patterning, enabling leaflet emergence, while REDUCED COMPLEXITY inhibits growth locally around emerging leaflets, accentuating shape differences created by patterning. We demonstrate the predictivity of our findings by reconstructing key features of C. hirsuta leaf morphology in A. thaliana.
- Mosca, G., Adibi, M., Strauss, S., Runions, A., Sapala, A., Smith, R. S. Modeling plant tissue growth and cell division. Mathematical Modelling in Plant Biology (Springer International Publishing), 2018. doi: 10.1007/978-3-319-99070-5
Morphogenesis is the creation of form, a complex process requiring the integration of genetics, mechanics, and geometry. Patterning processes driven by molecular regulatory and signaling networks interact with growth to create organ shape, often in unintuitive ways. Computer simulation modeling is becoming an increasingly important tool to aid our understanding of these complex interactions. In this chapter we introduce computational approaches for studying these processes on spatial, multicellular domains. For some problems, such as the exploration of many patterning processes, simulation can be done on static (non-growing) templates. These can range from abstract idealized cells, such as rectangular or hex grids, to more realistic shapes such as Voronoi regions, or even shapes extracted from bio-imaging data. More dynamic processes like phyllotaxis involve the interaction of growth and patterning, and require the simulation of growing domains. In the simplest case growth can be modeled descriptively, provided as an input to the model. Growth is specified globally, and must be designed carefully to avoid conflicts (growing cells must fit together). We present several methods for this that can be applied to shoots, roots, leaves, and other plant organs. However, when shape is an emergent property of the model, different cells or areas of the tissue need to specify their growth locally, and physically-based methods (mechanics) are required to resolve conflicts. Among these are mass-spring, finite element, and Hamiltonian-based approaches.
- Sapala, A.*, Runions, A.*, Routier-Kierzkowska, A. L.*, Das Gupta, M., Hong, L., Hofhuis, H., Verger, S., Mosca, G., Li, C. B., Hay, A., Hamant, O., Roeder, A. H. K., Tsiantis, M., Prusinkiewicz, P., Smith, R. S., Why plants make puzzle cells, and how their shape emerges. eLife, 2018; 7:e32794. doi: 10.7554/eLife.32794
The shape and function of plant cells are often highly interdependent. The puzzle-shaped cells that appear in the epidermis of many plants are a striking example of a complex cell shape, however their functional benefit has remained elusive. We propose that these intricate forms provide an effective strategy to reduce mechanical stress in the cell wall of the epidermis. When tissue-level growth is isotropic, we hypothesize that lobes emerge at the cellular level to prevent formation of large isodiametric cells that would bulge under the stress produced by turgor pressure. Data from various plant organs and species support the relationship between lobes and growth isotropy, which we test with mutants where growth direction is perturbed. Using simulation models we show that a mechanism actively regulating cellular stress plausibly reproduces the development of epidermal cell shape. Together, our results suggest that mechanical stress is a key driver of cell-shape morphogenesis.
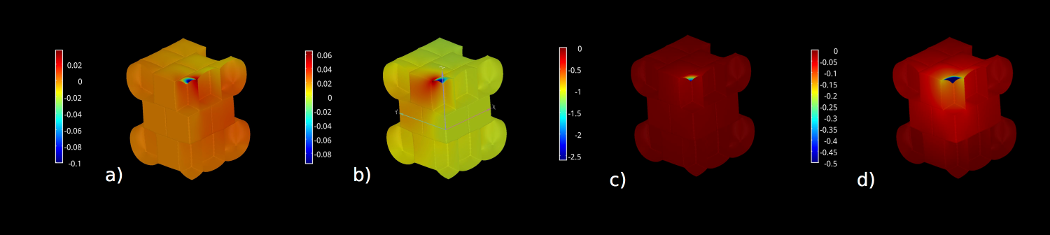
- Mosca, G., Sapala, A., Strauss, S., Smith, R. S., On the micro-indentation of plant cells in a tissue context. Phys. Biol., 2017 Feb 9;14(1):015003. doi: 10.1088/1478-3975/aa5698
The effect of geometry on cell stiffness measured with micro-indentation techniques has been explored in single cells, however it is unclear if results on single cells can be readily transferred to indentation experiments performed on a tissue in vivo. Here we explored this question by using simulation models of osmotic treatments and micro-indentation experiments on 3D multicellular tissues with the finite element method. We found that the cellular context does affect measured cell stiffness, and that several cells of context in each direction are required for optimal results. We applied the model to microindentation data obtained with cellular force microscopy on the sepal of A. thaliana, and found that differences in measured stiffness could be explained by cellular geometry, and do not necessarily indicate differences in cell wall material properties or turgor pressure.
- Hofhuis, H.*, Moulton, D.*, Lessinnes, T.*, Routier-Kierzkowska, A. L.*, Bomphrey, R. J.*, Mosca, G., ... & Ventikos, Y., Morphomechanical Innovation Drives Explosive Seed Dispersal. Cell. 2016 Jun 30;166(1):222-33. doi: 10.1016/j.cell.2016.05.002.
How mechanical and biological processes are coordinated across cells, tissues, and organs to produce complex traits is a key question in biology. Cardamine hirsuta, a relative of Arabidopsis thaliana, uses an explosive mechanism to disperse its seeds. We show that this trait evolved through morphomechanical innovations at different spatial scales. At the organ scale, tension within the fruit wall generates the elastic energy required for explosion. This tension is produced by differential contraction of fruit wall tissues through an active mechanism involving turgor pressure, cell geometry, and wall properties of the epidermis. Explosive release of this tension is controlled at the cellular scale by asymmetric lignin deposition within endocarp b cells—a striking pattern that is strictly associated with explosive pod shatter across the Brassicaceae plant family. By bridging these different scales, we present an integrated mechanism for explosive seed dispersal that links evolutionary novelty with complex trait innovation.
- Weber, A., Braybrook, S., Huflejt, M., Mosca, G., Routier-Kierzkowska, A. L., Smith, R. S., Measuring the mechanical properties of plant cells by combining micro-indentation with osmotic treatments. J. Exp. Botany 66(11) (2015): 3229-3241. doi: 10.1093/jxb/erv135
 Growth in plants results from the interaction between genetic and signalling networks and the mechanical properties of cells and tissues. There has been a recent resurgence in research directed at understanding the mechanical aspects of growth, and their feedback on genetic regulation. This has been driven in part by the development of new micro-indentation techniques to measure the mechanical properties of plant cells in vivo. However, the interpretation of indentation experiments remains a challenge, since the force measures results from a combination of turgor pressure, cell wall stiffness, and cell and indenter geometry. In order to interpret the measurements, an accurate mechanical model of the experiment is required. Here, we used a plant cell system with a simple geometry, Nicotiana tabacum Bright Yellow-2 (BY-2) cells, to examine the sensitivity of micro-indentation to a variety of mechanical and experimental parameters. Using a finite-element mechanical model, we found that, for indentations of a few microns on turgid cells, the measurements were mostly sensitive to turgor pressure and the radius of the cell, and not to the exact indenter shape or elastic properties of the cell wall. By complementing indentation experiments with osmotic experiments to measure the elastic strain in turgid cells, we could fit the model to both turgor pressure and cell wall elasticity. This allowed us to interpret apparent stiffness values in terms of meaningful physical parameters that are relevant for morphogenesis.
Growth in plants results from the interaction between genetic and signalling networks and the mechanical properties of cells and tissues. There has been a recent resurgence in research directed at understanding the mechanical aspects of growth, and their feedback on genetic regulation. This has been driven in part by the development of new micro-indentation techniques to measure the mechanical properties of plant cells in vivo. However, the interpretation of indentation experiments remains a challenge, since the force measures results from a combination of turgor pressure, cell wall stiffness, and cell and indenter geometry. In order to interpret the measurements, an accurate mechanical model of the experiment is required. Here, we used a plant cell system with a simple geometry, Nicotiana tabacum Bright Yellow-2 (BY-2) cells, to examine the sensitivity of micro-indentation to a variety of mechanical and experimental parameters. Using a finite-element mechanical model, we found that, for indentations of a few microns on turgid cells, the measurements were mostly sensitive to turgor pressure and the radius of the cell, and not to the exact indenter shape or elastic properties of the cell wall. By complementing indentation experiments with osmotic experiments to measure the elastic strain in turgid cells, we could fit the model to both turgor pressure and cell wall elasticity. This allowed us to interpret apparent stiffness values in terms of meaningful physical parameters that are relevant for morphogenesis.
- Bassel, G. W., Stamm, P., Mosca, G., de Reuille, P. B., Gibbs, D. J., Winter,R., Janka, A., Holdsworth, M. J. and Smith R. S., Mechanical constraints imposed by 3D cellular geometry and arrangement modulate growth patterns in the Arabidopsis embryo. PNAS 111(23) (2014): 8685-8690. doi: 10.1073/pnas.1404616111
Morphogenesis occurs in 3D space over time and is guided by coordinated gene expression programs. Here we use postembryonic development in Arabidopsis plants to investigate the genetic control of growth. We demonstrate that gene expression driving the production of the growth-stimulating hormone gibberellic acid and downstream growth factors is first induced within the radicle tip of the embryo. The center of cell expansion is, however, spatially displaced from the center of gene expression. Because the rapidly growing cells have very different geometry from that of those at the tip, we hypothesized that mechanical factors may contribute to this growth displacement. To this end we developed 3D finite-element method models of growing custom-designed digital embryos at cellular resolution. We used this framework to conceptualize how cell size, shape, and topology influence tissue growth and to explore the interplay of geometrical and genetic inputs into growth distribution. Our simulations showed that mechanical constraints are sufficient to explain the disconnect between the experimentally observed spatiotemporal patterns of gene expression and early postembryonic growth. The center of cell expansion is the position where genetic and mechanical facilitators of growth converge. We have thus uncovered a mechanism whereby 3D cellular geometry helps direct where genetically specified growth takes place.