Current Research
I am working in the field of morphodynamics and my research focuses on mechanical modeling of plant morphogenesis.
For this purpose I developed and keep expanding a computer simulation software based on FEM (www.morphomechanX.org) that allows to perform both steady state and growth mechanical simulation on realistic templates obtained either from confocal microscopy or custom designed (the software is integrated in the image processing platform MorphoGraphX, de Reuille et al.). The mechanical simulations can be complemented with genetic signaling networks to study the joint interaction of geometry, mechanics and genetics.
Modeling of Morphogenesis
I am interested in shape formation, especially in how symmetry is broken during early organ development, what is the contribution of mechanics and geometrical cues to this process and what stabilises forming patterns and shapes with respect to perturbations (for example stress feedback based growth).
Since plants grow symplastically and cells can not slide one past each other, differences in locally specified growth need to be accomodated by elastic deformations in the overall tissue, so that the resultant shape and its mechanical condition (state of residual stresses and strains) can normally not be derived intuitively or with purely analytical tools, but numerical simulations are required.
In the previous animation we see a cylindrical shell growing accordingly to a growth rule which increases non-linearly along the height of the cylinder. At a certain point of growth, due to buckling , the model stops being axisymmetric. For rendering purposes I recorded also non-equilibrium configurations and the colorbar represents the trace of Cauchy stresses which for each frame are rescaled based on maximal and minimal values.
In collaboration with Richard Smith and Brendan Lane at the MPI in Cologne, I am developing in silico tools to model mechanically based organogenesis at the continuous tissue limit (where the individual cells are not represented) and at cellular resolution . The two modeling techniques are complementary and can be used on the same biological sample. This allows to connect local and global mechanical properties of the tissue during growth and to predict how these interact with genetic signaling in order to shape the emerging organ/tissue (as an example see http://www.pnas.org/content/111/23/8685.full).
Currently my subject of study in Celia Baroux Group is the early ovule development in planta (A.thaliana and Maize). My aim is to characterise first the pattern of cell growth and proliferation in the ovule, with specific focus to the events leading to the Mega-Spore-Mother-Cell formation. Afterwards by combining mechanical modeling with imaging data (obtained by partner colleagues) I want to elucidate what is the contribution of mechanical cues (together with genetic signaling), to the ovule growth and patterning.
This study is part of the IMAGO project.
Characterisation of mechanical properties of plant tissues
Quantification of the steady-state mechanical properties of plant tissues is fundamental in order to represent properly the kind of stresses cells are undergoing or reciprocally exerting within the tissue itself. This is turn can provide essential informations about specific organs functionalities (es. stomata, seed valves). By observing how those properties change during organ growth we can as well infer what mechanical-physiological processes are occurring.
Cellular Force Microscopy techniques combined with plasmolysis experiments provide an essential tool for this task, but the results of those experiments provide data which are a combination of turgor pressure, mechanical properties of the cell wall and geometrical constraint. To interpret those data it is essential to use reverse engineering method in order to fit the parameters ( so we simulate the inflation of the organ and the subsequent indentation with CFM with an initial guess of the parameters and try to match the experimental results).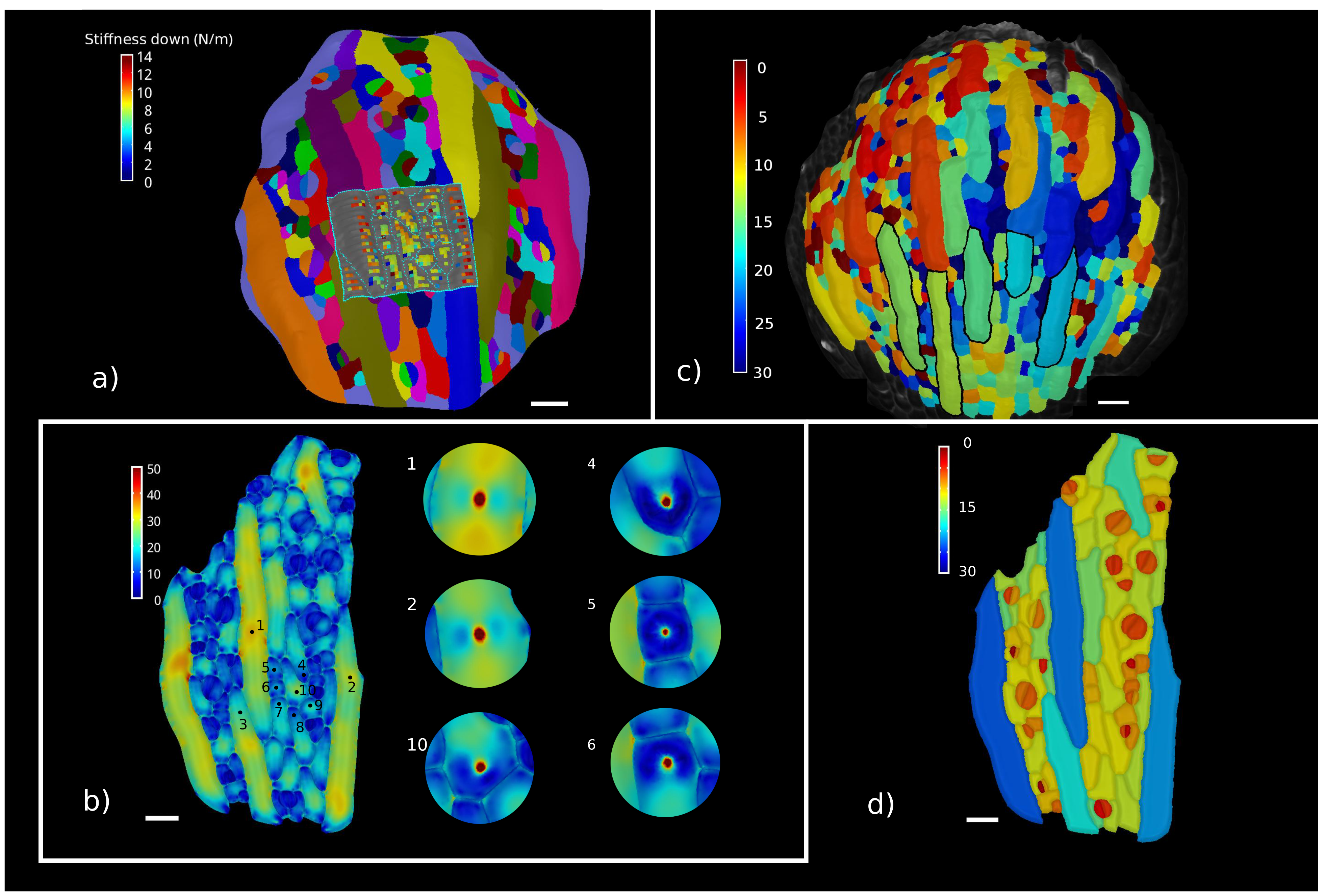
Example of reverse engineering approach to fit pressure and material properties in sepal cells of A.thaliana. The top panel shows experimental results of CFM and plasmolysis experiments, while the bottom layer shows how those results were reproduced in simulations (Phys. Biol., 2017 Feb 9;14(1):015003. doi:10.1088/1478-3975/aa5698).